Fluoride, as an essential trace element in the human body, can not only prevent dental caries but also promote bone metabolism. However, excessive intake of fluoride is also harmful to human health. Long term excessive intake of fluoride can lead to dental fluorosis; Due to the bone friendly properties of fluoride, it can also alter the bone density of the human body, and in severe cases, lead to fluorosis. The World Health Organization lists fluorine as the third largest pollutant absorbed by the human body that causes major diseases, after arsenic and nitrate.
As one of the main sources of drinking water, the fluoride content in groundwater determines people's health status. Fluorine pollution in groundwater is mainly caused by two factors: natural and anthropogenic. Natural pollution refers to the dissolution of fluorine-containing minerals such as fluorapatite and mica through rainfall, causing fluorine to continuously infiltrate into the soil, resulting in an increase in fluoride content in groundwater. Human pollution refers to the discharge of fluorine-containing wastewater during industrial production, including glass, electroplating, semiconductor processing, aluminum manufacturing, etc., which enters the ecological environment and seeps into the soil. Fluoride ions continue to accumulate, leading to groundwater pollution. The fluoride content in wastewater discharged from the glass manufacturing industry can reach 194-1980mg/L, and the fluoride content in wastewater discharged from phosphate fertilizer plants can be as high as 1500mg/L. In addition, fertilizers and pesticides used in agricultural production contain a large amount of fluoride, which can flow into the soil with rainwater and contaminate groundwater after enrichment. Fluoride pollution in groundwater has become a global environmental pollution problem. In order to protect the human living environment, reduce the incidence of endemic fluorosis, and control the fluoride content in drinking water, it is of utmost importance.
Current situation and challenges in the treatment of fluorine-containing wastewater
Domestic and foreign scholars have conducted extensive research on the treatment of fluorine-containing wastewater, using commonly used methods such as precipitation, ion exchange, electrochemical, and membrane separation.
(1) Chemical precipitation method is the most commonly used method for treating fluorine-containing wastewater [12]. It mainly involves adding a precipitant to the wastewater to convert fluoride ions into insoluble precipitates or form co precipitates through complexation. The purpose of removing fluoride ions is achieved through solid-liquid separation, such as calcium salt precipitation. The most commonly used method for Ca2++F-CaF2 is to add calcium salts such as lime and carbide slag as precipitants to produce calcium fluoride precipitate by reacting fluoride ions with calcium ions. The CaF2 generated by this reaction is a colloidal precipitate that settles slowly, resulting in a long time required for the reaction to reach equilibrium, limited treatment depth, and inability to reduce the fluoride content in the effluent to below 1.5mg/L. In addition, the pH of the effluent after precipitation reaction is alkaline, requiring additional acid neutralization before discharge. The large amount of sludge generated by the reaction also increases the difficulty of subsequent treatment.
(2) Coagulation sedimentation method
The coagulation precipitation method refers to the addition of substances with coagulation ability or chemical reaction with fluoride ions, such as aluminum salts, iron salts, and other coagulants, to high concentration fluorine-containing water. The principle is that coagulants form a large number of positively charged colloidal particles in water, which adsorb negatively charged fluoride ions in the water. This method has the advantages of high efficiency, simple operation, and low power consumption, but it takes up a large area and the uncertainty of the dosage can easily lead to secondary pollution of the water body. [Al/Fe](OH)3+F-[Al/Fe](OH)2F+OH-
(3) Electrochemical fluoride removal can be divided into two categories: electrocoagulation and electrodialysis. Electrocoagulation is an electrolytic method in which an aluminum or iron electrode serves as the anode. Under the action of a direct current electric field on the surface, it hydrolyzes into different forms of hydroxides with coagulation properties, adsorbing fluoride ions and fluoride complexes in water to remove fluoride ions. The hydroxide produced by electrocoagulation method has high activity and the ability to remove fluoride ions. AlAl3++3eAl3++6H2O [Al (H2O) 6] Al3+(cathodic reaction) 2H++2eH2 (anodic reaction) Electrodialysis uses the potential difference as a driving force to make fluoride ions and cations flow through the ion selective exchange membrane to the anode and cathode respectively, thereby removing fluoride ions. This method does not require the addition of chemical agents and solves the problems of fluoride and salt removal simultaneously. However, due to the fact that the treatment system consists of multiple parts, the investment is large, the management is complex, and the energy consumption is high, which limits the widespread application of this method.
(4) Membrane separation method nanofiltration membrane is a membrane separation technology that emerged in the 1990s, named after the nanoscale pore size of the membrane. Nanofiltration is a novel membrane separation process driven by pressure, which falls between reverse osmosis and ultrafiltration. This method is particularly suitable for separating small molecule organic compounds and can fill the gap left by ultrafiltration and reverse osmosis. It can intercept small organic molecules and allow salt to pass through, requiring much lower external pressure than reverse osmosis membranes. Although nanofiltration membranes require low external pressure and minimal membrane fouling, the initial investment cost is high. Additionally, a certain amount of concentrated water is still produced, which requires subsequent treatment and hinders their further promotion and application. The actual fluoride content in fluoride containing wastewater is generally at the level of several μ g/L to several mg/L, in which there may be an indefinite amount of anions coexisting, such as Cl, NO3-, SO42-, etc. Conventional precipitation methods, electrochemical methods, membrane separation methods, etc. are difficult to meet the actual treatment needs of wastewater.
Industry customer demand
Enterprises producing fluorine-containing wastewater require an efficient, simple, and cost-effective method. The adsorption effect is directly related to the characteristics of the adsorbent. Conventional adsorbents have drawbacks such as low adsorption capacity and short service life. Domestic and foreign scholars have been committed to the development of new, efficient, and industrializable defluorination adsorbents for many years. The needs of waste production enterprises for the treatment of fluorine-containing wastewater include the following three points: (1) efficient and stable removal of fluoride from wastewater, and the treated effluent can meet the discharge standards; (2) Low investment cost, low operating cost, and convenient equipment operation and maintenance; (3) Advanced and reliable technology, with no secondary pollution.
Introduction to Haipu Customized Process
The principle of the Haipu adsorption process is to use the special adsorption materials developed by our company to selectively adsorb the components or substances to be removed. When the adsorption is saturated, a specific desorption agent is used to desorb the adsorption material, allowing it to regenerate. This process is continuously repeated. The conventional process diagram for treating wastewater by adsorption method is shown in Figure 4-1.
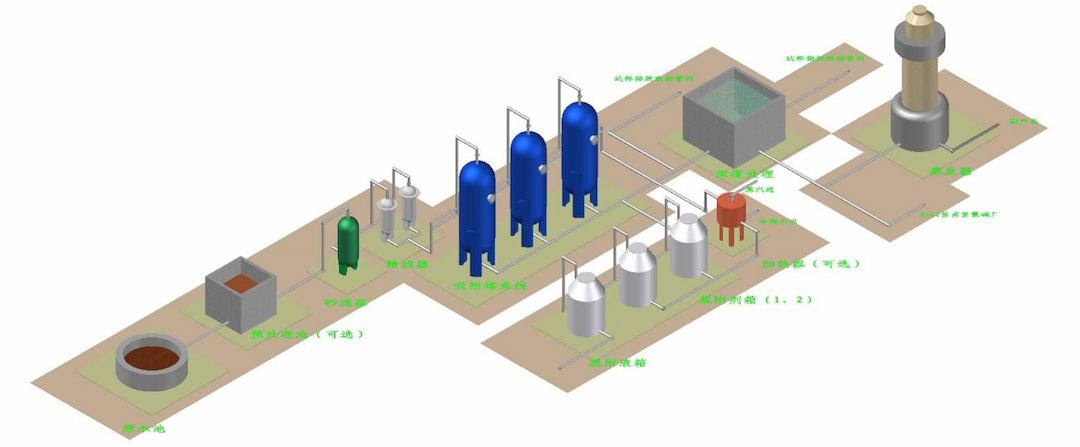
Figure 4-1 Conventional Process Diagram for Adsorption Treatment of Wastewater
When using Haipu's adsorption process to treat pyridine wastewater, the wastewater is pre filtered to remove suspended and particulate matter, and then enters the adsorption tower for adsorption. The special adsorption material filled in the adsorption tower can adsorb fluoride ions in the wastewater on the surface of the material. After adsorption saturation, the fluoride ions on the adsorbent material are first removed using sodium hydroxide solution, and the fluoride ions are transferred into the desorption solution. Then, a small amount of soft water is used to wash the alkaline solution remaining on the surface of the adsorbent material. Calcium chloride is added to the desorption solution and water washing solution to transfer the fluoride ions to the precipitate. The adsorption effluent is wastewater for removing fluoride ions, which can be directly discharged into the sewage treatment plant. The adsorption treatment process flow of fluorine-containing wastewater is shown in Figure 4-2.
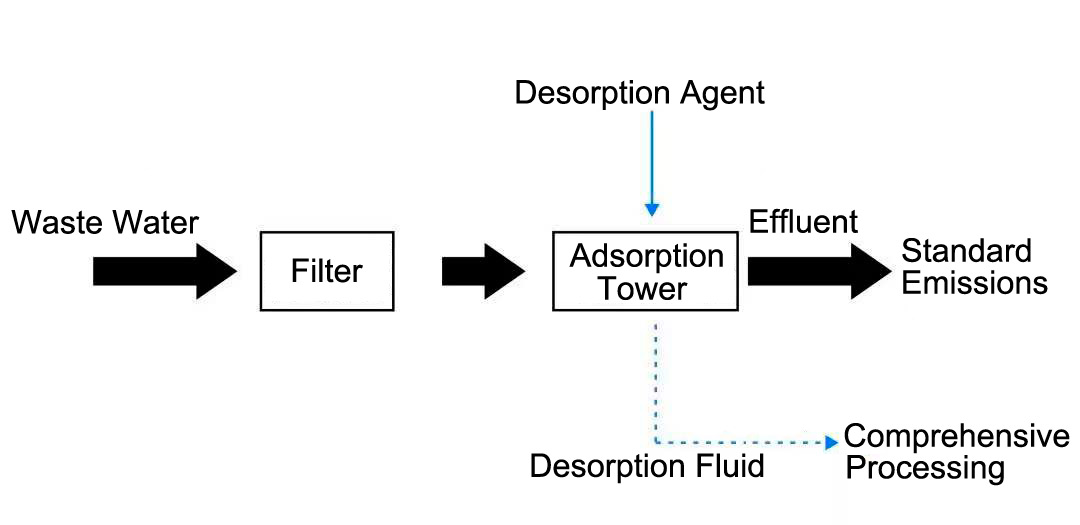
Figure 4-2 Fluorine Containing Wastewater Treatment Process Flow
Considering the fluctuation of fluoride ions in wastewater and the continuity of device operation, to ensure stable treatment effect, the industrial adsorption scheme requires the installation of three adsorption towers, two in series and one for desorption, that is, two in series for adsorption and one in rotation for desorption. Each time the front tower undergoes desorption, the desorbed tower is connected in series with the adsorption stage. Before desorption, the three towers are switched through pipeline valves to achieve different operating states, allowing each tower to circulate sequentially between the desorption, front tower, and rear tower processes. The illustrated process is shown in Figure 4-3, where each adsorption tower rotates its roles in the order of arrows during different operating periods.
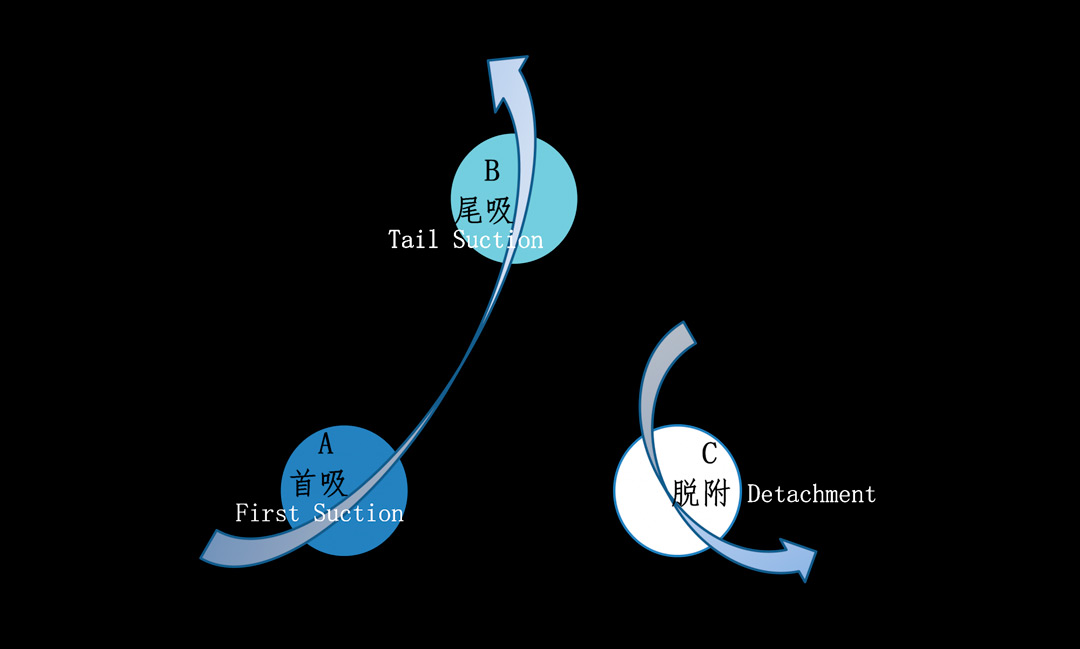
Figure 4-3 Schematic Diagram of the Operation Process of Series Adsorption (2 Adsorption and 1 Desorption)
Process treatment effect
The use of adsorption technology to treat fluorine-containing wastewater can effectively remove fluoride ions from the wastewater. The specific treatment data are shown in Tables 5-1 to 5-2.
A mining enterprise in Shandong produces groundwater with a fluoride content of about 1.4mg/L during the process of mining ore. The enterprise requires that the fluoride content in the treated wastewater be less than 0.5mg/L. Experimental treatment results show that using adsorption treatment can stabilize the removal rate of fluoride ions in the wastewater at over 92%, and the fluoride content in the effluent can be controlled below 0.5mg/L. While ensuring compliance with customer requirements, a certain safety margin is left, which can effectively prevent water quality fluctuations in the incoming wastewater from causing substandard effluent.
Table 5-1 Data on Fluoride Removal from Wastewater
| Raw Water Fluoride Content mg/L | Treated Water Fluoride Content mg/L | Removal Rate % |
| 1.4 | 0.04 | 97.14 |
| 1.4 | 0.10 | 92.86 |
| 1.4 | 0.08 | 94.28 |
A certain enterprise in Zhejiang produces wastewater with a fluoride content of about 300mg/L, and requires that the fluoride content in the treated wastewater be less than 10.0mg/L. The experiment used adsorption method for treatment, and the removal rate of fluoride ions in the wastewater remained stable at over 96%. The fluoride content in the effluent was less than 10.0mg/L.
Table 5-2 Data on Fluoride Removal from Wastewater
| Raw Water Fluoride Content mg/L | Treated Water Fluoride Content mg/L | Removal Rate % |
| 300 | 9.10 | 96.97 |
| 300 | 9.50 | 96.83 |
| 300 | 9.40 | 96.87 |
Core advantages of craftsmanship
The advantages of adsorption method are as follows:
(1) Efficiently remove fluoride from wastewater with high removal efficiency, strictly control the concentration of fluoride in the treated wastewater, and keep the fluoride content below 0.5mg/L;
(2) Conduct experiments on sampling samples of wastewater generated on the enterprise site, based on technology, and design adsorption processes based on experiments. The matching degree between wastewater and processes is 100%;
(3) The equipment occupies less land, has a compact structure, and requires less investment in civil engineering and equipment; The desorption agent is applied multiple times and concentrated step by step, resulting in high drug utilization and low operating costs;
(4) It can be implemented in module component form, flexibly adjusted according to production capacity, and easy to install;
(5) Advanced and mature technology, no secondary pollution, strong technical support, and rich engineering application experience.

 CN
CN





