Preface
Ion exchange resin (IER) is a synthetic functional polymer material containing active groups, which is formed by introducing different types of ion exchange groups into cross-linked polymer copolymers. Ion exchange resin has functions such as exchange, selection, adsorption, and catalysis. In industrial wastewater treatment, it is mainly used to recover heavy metals and precious rare metals, purify toxic substances, and remove acidic or alkaline organic substances such as phenols, acids, amines, etc. from organic industrial wastewater.
The ion exchange resin method was once one of the most widely used technologies in industrial wastewater treatment in China. In the mid-1970s, Shanghai Guangming Electroplating Plant and others first applied ion exchange resin to treat chromium containing wastewater, achieving the triple purpose of eliminating pests, recovering chromic acid, and recycling a large amount of water. Since then, the ion exchange resin method has been widely used in the industrial wastewater treatment industry in large and medium-sized cities in China. In recent years, ion exchange method has received increasing attention due to its large processing capacity, ability to remove various metal ions and acid ions, good water quality, and reusability. It has become one of the main methods for treating heavy metal industrial wastewater.
At present, the ion exchange resins used can be divided into anion exchange resins, cation exchange resins, zwitterionic exchange resins, chelating resins, and redox type resins based on the properties of the functional groups they contain. The application of IER for industrial wastewater treatment and purification concentration not only enables resin regeneration, but also has simple operation, mature process conditions, and short process flow.
1. Definition and characteristics of chelating resin
Chelate resins are a type of cross-linked functional polymer material that can form multi coordination complexes with metal ions. The mechanism of chelating resin adsorbing metal ions is that the functional atoms on the resin undergo coordination reactions with the metal ions, forming a stable structure similar to small molecule chelates, while the mechanism of ion exchange resin adsorption is electrostatic interaction. Therefore, compared with ion exchange resins, chelating resins have stronger binding affinity and higher selectivity with metal ions, and are widely used in the recovery and separation of various metal ions, the separation of amino acids, and wet metallurgy. It has good processing effect, stable adsorption and desorption performance, good reusability, and is characterized by simple operation and no secondary pollution.
2. The significance of chelating resin in purifying lithium solution
With the promotion and application of lithium batteries in new energy electric vehicles, the demand for lithium carbonate in China's market is growing rapidly. The purity of lithium carbonate has a significant impact on the performance of lithium batteries, but as a major producer of lithium carbonate, China is unable to meet the growing demand for high-purity lithium carbonate domestically. Every year, a large amount of high-purity lithium carbonate is imported into Japan, which greatly hinders the development of the domestic new energy industry. The main purpose of using ion exchange systems in purification production is to remove impurities. The concentration of monovalent ions in the feed solution is high, and in order to eliminate monovalent ion interference, the ion exchange resin used has strong selectivity for divalent ions. The exchange efficiency of traditional cation exchange resins is easily affected. Chelating resins are selected, which can selectively adsorb alkaline earth metal (calcium, magnesium, strontium, barium, etc.) ions and other divalent and divalent metal ions from alkali metal (lithium sodium potassium) salt solutions.
3. The working mechanism of ion exchange resin
When raw water containing calcium and magnesium ions passes through the adsorption layer of ion exchange, the calcium and magnesium ions in the water undergo chelation reactions with the chelating resin, while releasing hydrogen ions. The water flowing out of the exchanger in this way removes the divalent ions from the water. When the chelating resin that adsorbs calcium and magnesium ions reaches a certain level, the hardness of the effluent increases. At this time, the ion exchanger automatically regenerates the adsorbent according to the predetermined program, using a higher concentration of acid solution to adsorb the saturated chelating resin and regenerate it to restore its adsorption performance.
4. Haipu Process Solution
Jiangsu Haipu Functional Materials Co., Ltd. is a high-tech enterprise dedicated to the research and development of high-performance adsorbents, catalysts, and their process applications. With a series of independently developed high-performance adsorbents and catalysts as the core, combined with independently developed process technology, Haipu has become a professional solution provider in the fields of environmental governance and resource recycling. At the same time, taking it as our responsibility to help industrial enterprises meet environmental standards and achieve sustainable development through resource utilization, we adopt modular lean production and develop engineering solutions based on research and development data. Relying on independently developed high-performance adsorbents and rigorous and comprehensive process development, Haipu has accumulated many treatment cases in wastewater treatment, solving development problems for many enterprises and creating value.
The company focuses on technological innovation, as well as quality, environmental, and occupational health and safety management. The company has passed the audit and certification of ISO9001 quality management system, ISO14001 environmental management system, and ISO45001 occupational health and safety management system, ensuring the provision of reliable and high-performance products and services to customers.
The Haipu team, based on the characteristics of specific heavy metal ions, utilizes the special functional groups of chelating resins to form complexes with heavy metal ions, which have high selectivity for adsorption and utilization of heavy metal ions.
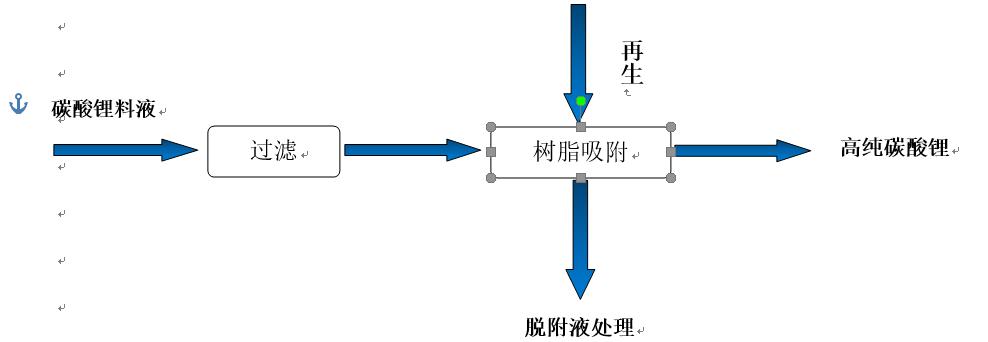
Figure 1 Process Plan Flow Chart
5. Process characteristics
The purification process technology is domestically leading, mature, reliable, economically practical, and stable in compliance with standards; The equipment designed and manufactured has the characteristics of progressiveness, high performance price ratio, high efficiency, high controllability, flexible adjustment, good start stop characteristics, long-term safe, economical, full load operation, and easy maintenance. Provide sufficient information to explain the technical conditions and data related to the control requirements, control methods, sequence control, interlock protection, and operating instructions for lithium liquid softening equipment.
Stable operation, the chelating resin is not prone to layer disorder, and the contact between the feed solution and the adsorbent is effective.
The chelating resin using a fixed bed is not easily broken and has low mechanical losses.
Adopting a unique operating mode, the ion exchanger is fully saturated and then cut out for regeneration, resulting in high utilization efficiency of the regeneration solution, complete regeneration, and saving regeneration solution.
Leave sufficient backwash space to thoroughly backwash the chelating resin.
Using chelating resin, only targeting divalent and above metal ions, without losing the lithium ion content in the feed solution.
After regeneration, set up flushing and configure online chloride ion monitoring instruments to monitor the flushing effect in real time, without introducing chloride ions into the system.
Set the top liquid step sequence for desalinated water, collect the material liquid by topping it out with desalinated water before use, regenerate it, and then return the topped out material liquid to the exchange column to ensure that lithium ions are not lost.
The process is controlled by a DCS upper computer, equipped with necessary online control instruments, and the entire process is supervised and controlled. Save manpower, facilitate operation, and supervise and control the entire process.

 CN
CN



