In 1817, Alphonsen discovered a new metal while analyzing lithium feldspar near Stockholm, and subsequently named the metal Lithium after his teacher, Swedish chemist Berzelius, with the element symbol Li (lithium). As the smallest atomic weight metal element, lithium has extremely strong electrochemical activity and highly reactive chemical properties. Therefore, lithium can easily react with other materials to form various alloys, which are widely used in various fields.
The content of lithium in the Earth's crust is about 0.0065% (approximately 600 trillion tons, although it is difficult for humans to fully exploit the entire crust), ranking 27th in the richness ranking. Although it is called a "rare metal", from the perspective of its natural content, it does not belong to the rare category. The reason why lithium is "rare" is not because of its stock, but because of its difficulty in purification.
At present, the level of technology makes a large amount of lithium minerals have no development value, such as lithium in seawater (the lithium reserves in seawater are about 260 billion tons), which is difficult to extract due to its low concentration. The industry consensus is that lithium can exist in both solid mineral resource states and liquid mineral deposit resource states. Solid lithium deposits exist in two forms: pegmatite type lithium deposits and sedimentary type lithium deposits. Liquid lithium deposits refer to brine type lithium deposits, mainly occurring in salt lake brine, seawater, oilfield brine, and well brine.
1. Salt Lake Lithium Resources and Development Status
There are two types of lithium deposits that can be developed and utilized globally: salt lake brine lithium deposits and rock lithium deposits. Salt lake brine lithium resources account for more than 70% of the total resources, mainly distributed in Chile, Bolivia, Argentina, China and other places.
Lithium salt lake resources in China are mainly distributed in Qinghai and Xizang. Among them, there are 10 lithium producing areas that have been included in the mineral reserves of Qinghai salt lake resources, and the reserves of lithium chloride are 24.4738 million tons. There are two super large mineral deposits in the Cha'erhan Salt Lake and Beiletan mining area, as well as three super large mineral deposits in the Xitai, Dongtai Jinnaer Lake, and Yiliping mining areas. The lithium resources in 10 salt lakes with lithium content reaching industrial grade amount to 8.92 million tons, which can be developed and utilized.
Salt lake resources in Xizang are mainly distributed in the northwest of Tibet. There are 80 salt lakes with lithium content of brine reaching the boundary industrial grade, including 8 large ones. The reserves of LiCl resources are 17.3834 million tons. The main mineral deposits include salt lakes such as Zabuye, Longmucuo, Jiezechaka, Laguocuo, and Eyacuo.
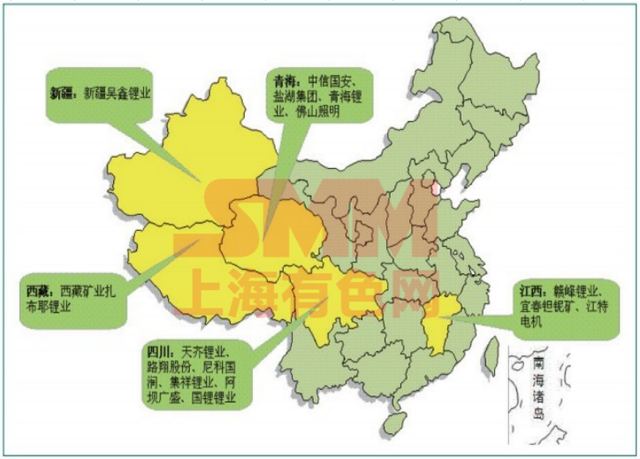
Figure 1 Distribution of lithium resources and development enterprises in some salt lakes
2. Development history and cost of lithium extraction technology from salt lakes
Before the 1960s, research on lithium extraction technology from brine had already begun, but most of it was only in the research and development stage and had not been put into practical application. After 1974, with the discovery of a large amount of lithium resources in salt lake brine, it boosted the development and investment enthusiasm of some major brine lithium resource countries and lithium mining development enterprises in the world. After 1980, companies such as Fute and FMC in Cyprus began to aggressively enter the field of salt lake lithium extraction, marking the beginning of the industrialization of lithium extraction from salt lake brines. The lithium extraction technology from salt lakes mainly includes precipitation method, solar cell method, extraction method, calcination method, Mo method, adsorption method, and other new lithium extraction technologies.
2.1 Precipitation method
The precipitation method is only applicable to salt lake brines with Mg 2+/Li+less than 10, so this technology has been applied in salt lakes with low Mg 2+/Li+such as Atacama, Yinfeng, and Umbre Muerto in South America. This technology can achieve magnesium lithium separation in the salt field process and concentrate Li+to above 30g/l. The subsequent process only requires deep impurity removal to meet production needs, and has the advantages of simple process technology, low energy consumption, and low investment.
Due to the above advantages, as early as 1986, the American company Fute in Cyprus stopped its domestic lithium extraction industry from spodumene and adopted this process to invest in the construction of lithium carbonate processing plants in Silver Peak, Nevada and Atacama Salt Lake in South America, opening the prelude to brine lithium extraction. In 1997, SQM successfully extracted lithium from the Atacama Salt Lake in Chile, reducing the price of lithium carbonate to $1500/t (compared to the international price of $3300/t during the same period), greatly impacting the hard rock lithium industry in countries around the world. In 2018, four salt lake lithium extraction companies, Yabao, SQM, Livent, and Orocobre, used this technology to produce a total of 158000 tons of lithium chemical products, accounting for 52.4% of the global lithium supply. The production cost was about 3000-5000 $/t, which has certain advantages in the industry. Due to the simplicity and maturity of this technology, the cost structure has remained largely unchanged in the past 40 years, with price changes mainly due to rising prices and increased labor costs.
2.2 Solar Pool Method
This process technology route is developed for carbonate salt lakes with extremely low (≤ 0.1) Mg2+/Li+ content. Due to the extremely low magnesium content in the brine of carbonate salt lakes, 60% and 70% of lithium carbonate crude ore can be obtained by directly drying the brine. In the later stage, only the lithium carbonate crude ore needs to be purified to obtain battery grade lithium carbonate products.
In March 2003, Zabuye Salt Lake started the construction of the first phase of lithium carbonate project using this technology, and successfully tested it in August 2005. However, due to the presence of carbonate ions in the salt lake, there is significant loss during the sun drying process. In addition, problems such as high altitude and oxygen deficiency above 4000 meters, as well as poor basic conditions, have limited the increase in production capacity. Currently, the designed production capacity is still 3000 tons per year, and a total of 2728 tons of lithium salt were produced in 2017. Due to the lack of technological innovation in the past decade, the cost structure has not undergone significant changes, and the current production cost is approximately 15000-20000 yuan.
2.3 Extraction method
The technology of solvent extraction for lithium extraction from brine has a history of more than 40 years, and has been studied by various institutions such as the American Lithium Corporation, the Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences, and the Qinghai Salt Lake Research Institute of the Chinese Academy of Sciences.
At present, the Dachaidan Salt Lake has adopted this technology and built an industrialized device, successfully producing lithium chloride and lithium carbonate. However, due to the high price of extractants, large losses, and equipment corrosion in the initial stage of this process, the production discontinuity was caused, resulting in a cost of up to 60000 yuan. In recent years, with the continuous advancement of this technology, the current production cost is about 40000 yuan.
2.4 Calcination method
The calcination method is a technology proposed for high magnesium lithium ratio salt lake brine. Due to the high magnesium lithium salt lake brine being sun dried and concentrated until the final brine (old brine) is a saturated solution of lithium rich brucite. Magnesium oxychloride decomposes into magnesium oxide and hydrogen chloride gas above 550, while lithium chloride does not decompose under these conditions. After calcination, the sintered material is leached. If lithium salt is easily soluble in water, it enters the solution. If magnesium oxide is almost insoluble in water, it remains in the slag. Impurities such as sulfate ions, magnesium, and a small amount of boron are present in the leaching solution. After purification of the filtrate, lithium carbonate product can be obtained by evaporation, alkali precipitation, and drying.
From 2007 to 2011, a calcination process production line was built in Xitaijiner Salt Lake. In the early stages of the project, due to issues such as equipment material selection, hydrogen chloride recovery, and magnesium slag recovery, production could not be continuous, resulting in a cost of over 80000 yuan. In 2015, with the booming lithium salt market, the company launched multiple technological research and technological transformation projects. After the completion of the renovation in 2016, the problems affecting the operation of the process have been improved, and the production cost has been reduced to about 40000 yuan (equivalent to the cost of most lithium extraction enterprises), especially the product quality has been greatly improved. Currently, the product has been applied to some large and influential battery material production enterprises such as Beijing Dangsheng Technology.
2.5 mo method
In 2007, a 3000TMO process production facility was successfully built in Dongtai Jinnaer Salt Lake. In the early stages of project completion, due to unstable device operation and high membrane consumption, the cost exceeded 60000 yuan. Later, with the mastery of electrodialysis membrane systems and the upgrading of electrodialysis membrane manufacturing technology, the problem of unstable device operation was gradually solved, and the production cost was significantly reduced to around 20000 yuan, which has a great cost advantage over lithium extraction technology by ore method. In 2012, a new 7000 ton production line was successfully built and put into operation. After several years of improvement and renovation, the two production lines have a total production capacity of 10000 tons. Currently, the two production lines can produce a main content greater than 99.6% at once, and the product quality fully meets the local standard for brine battery grade lithium carbonate. 90% of the products are sold to battery material manufacturers, and representative downstream customers include Peking University Pioneer, Tianjin Bamo, Shanshan, and Beijing Dangsheng.
At present, the newly built 10000 ton production line of Qinghai Dongtai Jinnaer Lithium Resources Co., Ltd. has been completed and put into operation. As of now, the capacity of industrialized facilities built using this technology has reached 20000 tons per year.
2.6 Adsorption method
Compared to other methods, adsorption is a simple and efficient approach, and the key to lithium extraction in this method is high-performance adsorbents. At present, most of the research reports at home and abroad are on inorganic adsorbents, which utilize the special internal structure of adsorbents to block larger alkali metal and alkaline earth metal ions during lithium adsorption, thereby achieving efficient screening of magnesium and lithium. But these inorganic adsorbents are mostly in powder form, with small particle size, poor mechanical strength, flowability, and permeability, and high adsorbent loss rate.
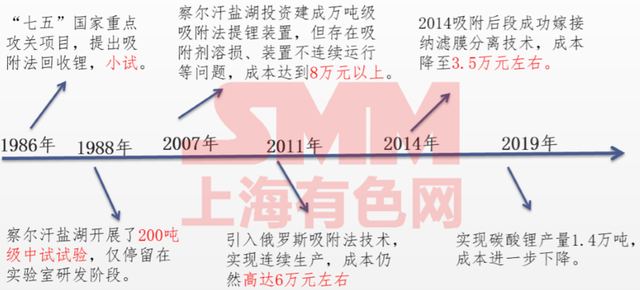
Figure 2 Time roadmap for lithium extraction by adsorption method in China
3. Haipu special lithium extraction adsorbent
To solve the problems of low capacity, poor mechanical strength, and high dissolution rate of existing adsorbents in the market, we have developed a new lithium extraction adsorbent material DL760.
By synthesizing nano active lithium extraction particles and using special granulation technology, it is ensured that the active nanoparticles do not aggregate or become inactive, and still have nanometer size; At the same time, the lithium extraction adsorbent has excellent mechanical strength, thereby ensuring the lithium extraction activity, capacity, and stability of the lithium extraction adsorbent.
As shown in the figure, the left image is an electron microscope image of the appearance of DL760, and the right image is a further magnified lens image of the nanoparticle.

Figure 3: Appearance of DL760 (left), High Power Transmission Electron Microscope (right)
It can be seen that the active lithium extraction nano material has good nano size and dispersibility, which can maintain nano activity and stability. The adsorbent has excellent mechanical strength, low dissolution rate, and is suitable for various magnesium lithium ratio brines. It also has high lithium extraction yield, green environmental protection, no secondary pollution, and low operating costs.
The production process of lithium carbonate with special lithium adsorbent DL760 adsorption as the core includes four steps: lithium extraction, deep magnesium removal, concentration, and precipitation.
The first step of lithium extraction by adsorption is the core and key technology of salt lake lithium extraction, which separates and extracts lithium from brine with high magnesium lithium ratio.
Subsequent deep magnesium removal can be achieved by using magnesium removal adsorbents to remove a small amount of magnesium deeply; Concentration can be achieved through reverse osmosis, followed by further concentration through multi effect evaporation; Finally, lithium carbonate product was obtained by precipitation with sodium carbonate, as shown in the figure:
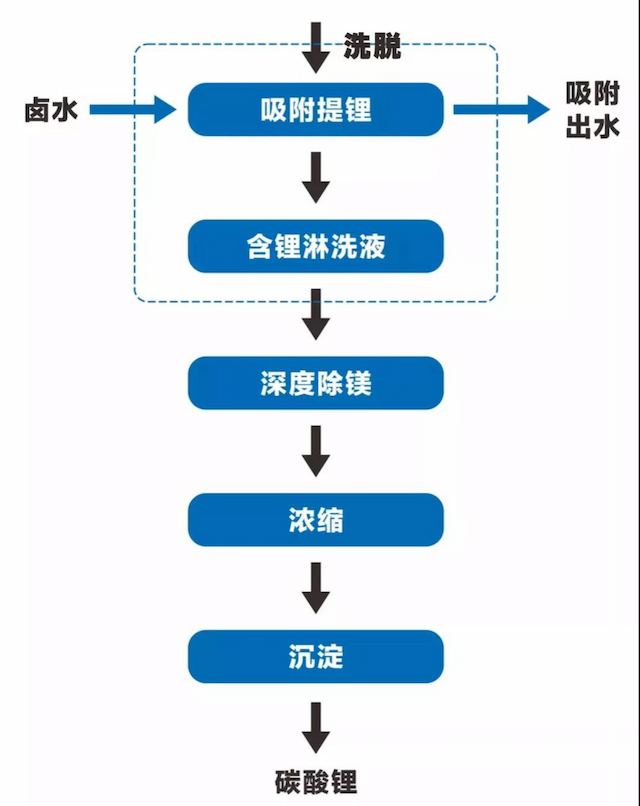
3.1 Industrialization Status
Based on the preliminary small-scale, pilot scale, and hundred kilogram scale trial production, as well as the development of related application processes, the production and industrial application of a ten ton new lithium extraction adsorbent have been completed. A lithium extraction trial production device with a daily processing capacity of 100 tons of brine has been established at a salt lake site in Qinghai.
Further carry out industrial application research, simulate industrial application scenarios, optimize operating process conditions, investigate the performance and stability of new lithium extraction adsorbents, and prepare for subsequent industrial scaling up.
The following table shows that after 6 months of continuous operation, all technical indicators have met the requirements and are suitable for lithium extraction from various brines with lithium content of 50-2500mg/L and the highest magnesium content in a saturated state, achieving efficient lithium magnesium separation.
Table 1 Technical indicators and parameters related to the new lithium extraction adsorbent
| Serial number | Index | Removal rate |
| 1 | Adsorption temperature ≤ 40 ℃ | 25-40℃ |
| 2 | Desorption temperature ≤ 50 ℃ | 25-45℃ |
| 3 | Lithium content in lithium leaching solution ≥ 1g/L | 1.0-1.2g/L |
| 4 | Magnesium content in lithium leaching solution ≤ 3g/L | 1.5-3.0g/L |
| 5 | Lithium adsorption rate ≥ 90% | 90-95% |
4. Outlook
In the past decade, although high magnesium lithium ratio salt lake lithium extraction technology has achieved great results, there is still a lot of room for improvement in production costs compared to low magnesium lithium ratio salt lake lithium extraction in South America. In the future, with the acceleration of lithium resource development and the release of production capacity, as well as other factors, if market prices continue to decline, many high cost and resource scarce enterprises will find it difficult to sustain themselves, and may even suffer losses and production shutdowns.
In order to achieve sustainable development in the fierce market competition, increasing technological investment, improving and optimizing processes, enhancing product quality, and reducing production costs are issues that every high magnesium lithium ratio salt lake lithium extraction enterprise needs to consider.

 CN
CN






